Web-based biosafety software that handles the time-intensive tasks of managing Biosafety protocols – Simply.
Protocol Summary Document
Save time by consolidating all your information into a useful piece of information – the Protocol Summary. This snapshot includes the essential components that you are tracking (Agents, Personnel, Locations, etc.). This information is pulled directly from the data that you enter as you are managing the protocol.
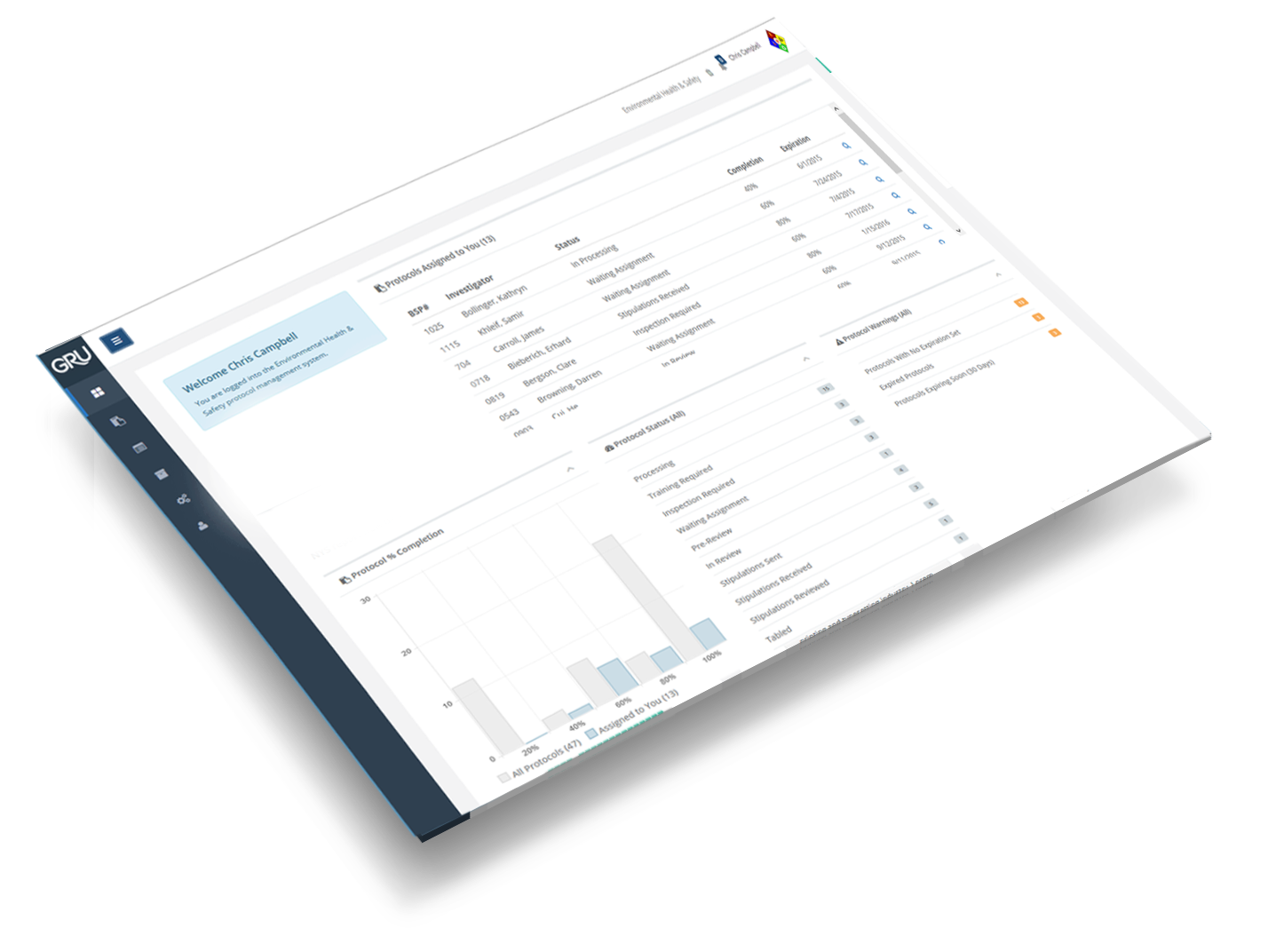
Data Organization
Data is organized in the Protocol Workspace to manage documents and data, a workspace for Inspections, and a document library to store the approved protocol and supporting documents. An uncluttered design allows for less complication.
Instant Access
You are able to get to protocol information easily and quickly through Google-like searching of data and documents.
Discover more great features
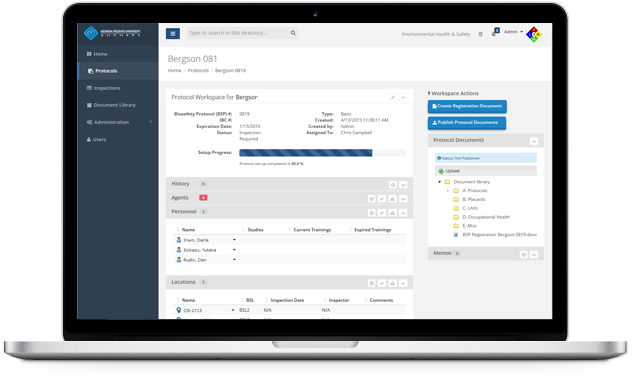
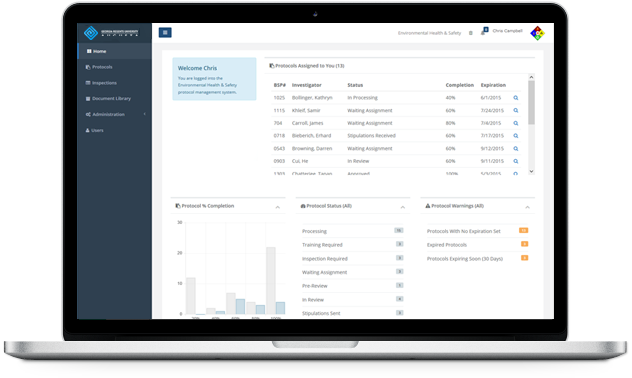
Protocol Workspaces
Development and collaboration for a protocol takes place in a central area known as a Protocol Workspace. You or an assigned worker can perform tasks associated with managing the Protocol from new application to approval, and view the status and progress every step of the way.
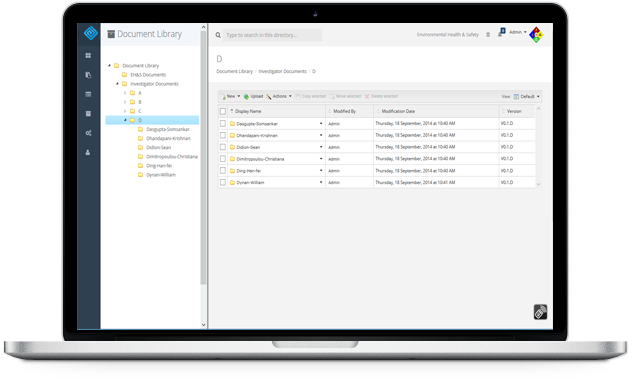
Document Library
Protocols and supporting documents are published to a document library which becomes part of the database. Once in place, it becomes trivial to find or check-out the documents that you need.
Inspection Workspaces
With each protocol comes the need for inspections. Like the Protocol, there is a workspace for inspections that can be used to organize reports and information needed for each laboratory biosafety inspection.
Searching
All documents and data entered into the system becomes immediately searchable, allowing you to find documents and to generate reports, making “needles” easier to find than you think!
Tablet Friendly
It is not practical to think that you will always be at your desk when information needs to be entered for a given protocol. Having a tablet friendly design gives you the chance to enter information on the spot.
Want to see more? View our gallery for detailed screen shots.
We are happy to show off our software without scheduling a one-on-one demo.
